Week 8
After doing numerous tests, we have narrowed down the critical pressure of the glass tube. With this information, and the pending information from the leaking rate experiment that is till being run, we will be able to provide a shelf life for the VacuStor tube. Now we need to determine the critical pressure of the plastic VacuStor tube as well. In addition, we will potentially explore the leaking rate at lower temperatures for the possibility of storing them at -4 or -20 degrees Celsius.
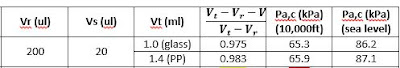
Critical Pressure Experimentation
As of now, we have determined the critical pressure to be -25 kPa under atmospheric pressure, or 76 kPa. Anything past this critical pressure will not draw enough sample for proper analysis. The minimum sample required is 90 microliters, so 76 kPa internal pressure is enough to draw that much. However, the optimal pressure is any pressure lower than -30 kPa below atmospheric pressure, or 71 kPa. This ensures 100% sample transfer required for proper assay analysis and accurate results. The pressure determined seems to be accurate
Once we obtain the leaking rate time to reach this pressure, we will know the shelf life of the tube at room temperature. However, we also know pressure and leaking rate of pressure is not only dependent on permeability, but also temperature. Hence, we are considering testing the leaking rate of the tube at different temperatures as well.
Effect of Temperature on Leaking Rate
A possible experiment I am considering is to determine the effect of temperature on the leaking rate of the VacuStor tube. This may be helpful because if we can get a longer shelf life from keeping the tube at a lower temperature, it might be better to advise the tube be stored at lower temperatures if possible. So, in order to test this, we will evacuate a tube and then see how pressure and the vacuum inside is affected by the temperature.
I have begun to compile the already conducted experimental data and will hopefully begin to start my report.